Abstract: Petrochemical industries use bulk amount of mild steel in recovery of crude oil, refinery and transportation. Recovery of crude oil CO2 and SO2 gases is found into the well of petroleum. Large amount of saline water are used during recovery of crude oil in this water CO2 and SO2 are dissolved to produce H2CO3 and H2SO4. These acids create acidic environment of mild steel. Saline waters have possessed Cl– ions. It produces hostile environment for mild steel. Mild steel is protected by polymeric coating. But this coating is not shaved mild steel in moist air, sulphur dioxide and chloride ions environment. It produces chemical and electrochemical reaction with polymeric-coated metal and accelerates corrosion reaction. Chloride ions are entered inside by osmosis or diffusion process and develop corrosion cell on metal surface. Oxygen deficiency occurs inside and outside of polymeric-coated mild steel thus corrosion cell is automatically formed. These pollutants corrode polymer and metal in this ways start interior and exterior corrosion of polymeric-coated-metal. These pollutants rupture internal bond of polymer and produce disbonding between base metal and coating material. Metal exhibits various form of corrosion like galvanic, pitting, stress, crevice, blistering, embrittlement etc. Nanocoating and filler technique used to mitigate corrosion of materials in such corrosive environment. The composite barrier was synthesized by octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and MgS as filler. Corrosion rate of mild steel was determined by absence and presence coating and filler by gravimetric methods. Potentiostat used for calculation corrosion potential, corrosion current and corrosion current density. The composite barrier developed by the use of nozzle sprays. Langmuir isotherm and Arrhenius equation was used for the study of surface adsorption phenomenon. The confirmation of surface deposition and bond formation was studied by the use of activation energy, heat of adsorption, free energy, enthalpy and entropy. The experimental results surface coverage area and coating efficiency were shown that the used nanocoating and filler materials were developed composite thin film barrier on surface of mild steel.
Introduction
Petrochemical industries expense bulk amount of money for corrosion protection of materials. It is not fully control but its affect is controlled by the use of corrosion mitigation techniques. It is necessary for materials manufacturing industries to monitor carefully their internal morphology, shape and design. Materials can be synthesized (Bhadra S, 2011) as per need of surrounding environment (Szabo T, 2011) which does not change their physical, chemical and biological properties (Wen N T, 2008) and provide thermal stability, durability, capability, resistance power against corrosive medium (Boerio F J, 2005). Materials corrosion protection (Deveci H, 2012) check with application of coatings, inhibitors, sacrificial anodic protection and impressed current process are in ambient environment (Genzer J, 2005). There are various types of coating available like metallic, nonmetallic, polymeric and paint. Such coatings do not protect materials longer times. Inhibitors (Leon-Silva U, 2010) are used to control corrosion of metal in petroleum industries. Inhibitors are utilized in several forms (Baier R E, 2006) like organic, inorganic and mixed types inhibitors which are related to anodic as well as cathodic. Their application (Rao BVA, 2010) can be done in the form (Liu X Y, 2009) of solid, liquid and gas as requirement of corrosive medium. Electron rich compounds (Liao Q Q, 2009) alkane, alkene, alkyne, cyclic, aromatic and heterocyclic contain nitrogen, oxygen and sulphur are used as organic inhibitors in petroleum industries (Zhang D Q, 2009), sugar industries (Sahoo R R, 2009), phosphate industries (Raman R, 2007), pulp and paper industries (Li D G, 2006) to control the corrosion of mild steel (Cristiani P, 2008) and stainless steel (Cristiani P, 2005). These inhibitors do not provide protection longer periods (Videla H, 2009). Metallic pipe corrosion (Bibber J W, 2009) is mitigated by anodic protection and impressed current but they do not give good results in aggressive medium (Ghareba G S, 2010). Aloe Vera is used check corrosion metallic can (Singh R K, 2016) which contain beverages, orange juice, milk and vegetables. It works as natural inhibitor. In acidic soil the life of earthworms (Singh R K 2017) become miserable such environment their life can be protected by the application aloe vera juice. Human skin is face corrosion problem in mega and metro cities environment such corrosion is control by the use of aloe vera and turmeric coating. Nanocoating and filler compounds (Singh R K, 2017) are used to control the corrosion of polymeric-coated metal in ambient of effluents.
Experimental
Mild steel sample (4X8X0.1) cm2 was taken for experimental work. It was coated with polybutadiene and kept in SO2 moist and Cl– ions environment. The corrosion rate of samples were determined by gravimetric technique at 283, 293, 303, 312 and 3230K temperatures and that temperatures exposer times were 3, 5, 8, 11 and 14days. These samples were nanocoated with octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and corrosion rate calculated at above mentioned temperatures and days. Nanocoated samples were again coated with MgS and measured corrosion rates. Potentiostat technique used to calculate corrosion potential, corrosion current, corrosion current densities of sample without and with nanocoating above mentioned materials. Potentiostat consists pt electrode used as reference electrode, calomel as auxiliary electrode and polybutadine-coated mild steel sample electrode and this electrode can be with octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and coating with MgS. Nanocoated compound octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone was synthesized by given methods as:
Scheme1: Synthesis of 4-chloro-1, 2-dihydonaphthalene
Phosphorous pentachloride (30g) treated with 3, 4-dihydronaphthalen-1(2H)-one (25gm) and then after benzene (50gm) added, the reaction mixture was stirred for one hour. The reaction mixture was quenched with NaHCO3 and did workup with diethyl ether. The solvent evaporated with rotator vapour. The product was purified by silica gel column chromatography and produced 89% 4-chloro-1, 2-dihydonaphthalene.

Physical properties of 4-chloro-1, 2-dihydronaphthalene

H1NMR of 4-chloro-1, 2-dihydronaphthalene

Scheme2: Synthesis of 1, 2-didehydro-3,4-dihydronaphthalene
4-Chloro-1, 2-dihydronaphthalene (10gm) kept in two neck round bottle flask and potassium t-butaoxide (25gm) dissolved in THF solution. This solution poured into 4-Chloro-1,2-dihydronaphthalene and reaction temperature 0oC. The reaction was mixture stirring four hours after completion reaction added cyclohexene as trapping agent and again stirring reaction more two hours. After work up got adduct 90% of 1,2-didehydro-3,4-dihydronaphthalene.

Physical properties of 1,2-didehydronaphthalene

Scheme3: Synthesis of benzo-decahydrobiphenylene
When 1,2-didehydro-3,4-dihydronaphthalene was used with cyclohexene, it was trapped by 1,2-didehydro-3,4-dihydronaphthalene to yield benzo-decahydrobiphenylene.
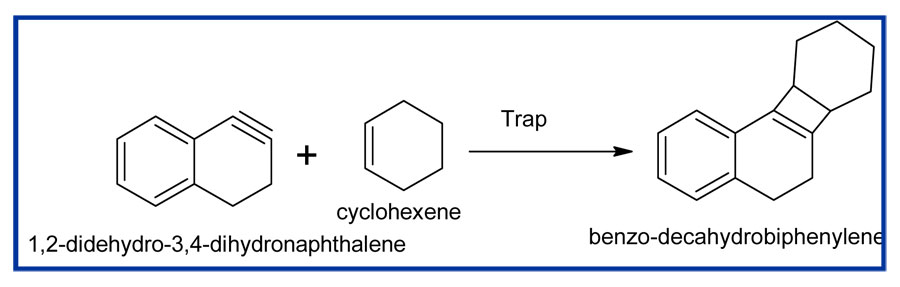
Physical properties of benzo-decahydrobiphenylene

H1NMR of benzo-decahydrobiphenylene

Scheme4: Synthesis of octahydrodibenzo[a,d][8]annulene-5,12-dione
Adduct (20gm) oxidized into benzo-decahydrobiphenylene with addition of NaIO4 (10gm) and RuO2 (15g) in the presence of solvent CH3CN and CCl4. The reaction was quenched with H2O and after workup 87% yield of octahydrodibenzo[a,d][8]annulene-5,12-dione was obtained.
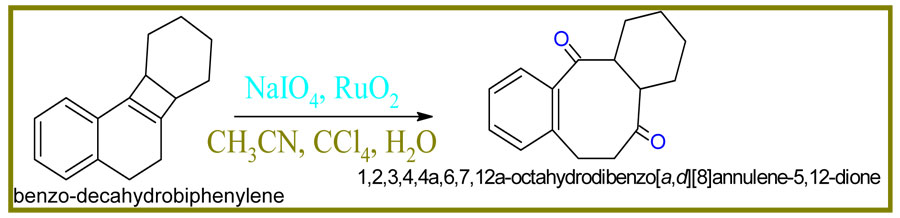
Physical properties of octahydrodibenzo[a,d][8]annulene-5,12-dione

H1NMR of octahydrodibenzo[a,d][8]annulene-5,12-dione

Scheme5: Synthesis of octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone
Octahydrodibenzo[a,d][8]annulene-5,12-dione (35g) was taken in a round bottomed flask and 75g of hydrazine hydrate was added and the mixture was heated under reflux for 24 hours. The solution was cooled in an ice bath and the octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone was separated by suction filtration. The crystals were washed with 150ml of cold ethanol and dried on the suction filter for 1 hour. The yield of octahydrodibenzo[a,d][8]annulene-5,12-dioxime 90% was obtained.
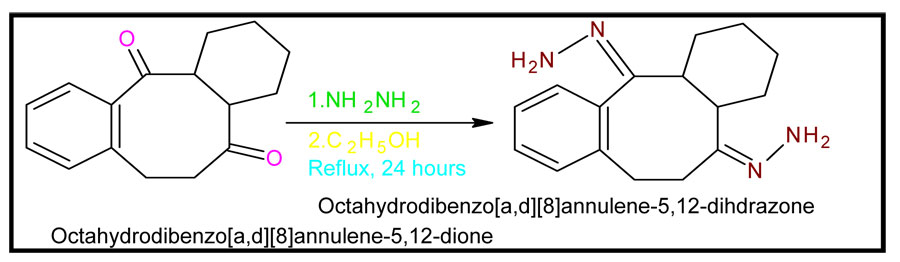
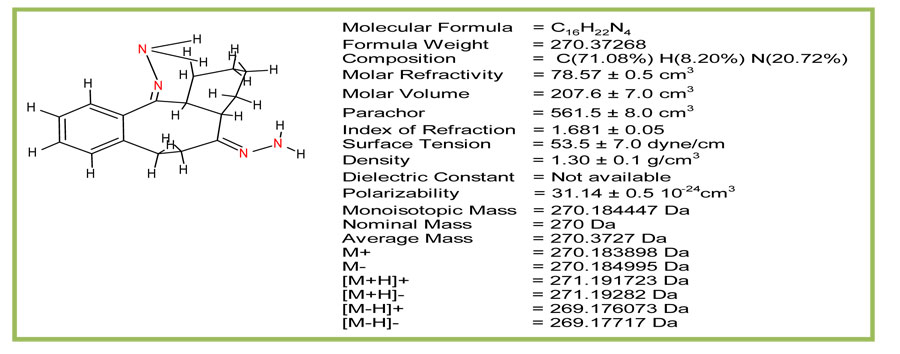
Physical properties of octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone

Results and Discussion
Octahydrobenzo[a,d][8]annulene-5,12-dihydrazone was nanocoated on the surface of polybutadiene-coated mild steel and their porosities were blocked by MgS filler. The corrosion of metal was determined in marine water in three stages, one polybutadiene-coated mild steel, second nanocoated octahydrobenzo[a,d][8]annulene5,12-dihydrazone and third was coated with MgS. The corrosion rate of each material was calculated by weight loss formula K(mmpy) = 13.56 W / D A t (where W = weight loss of test coupon expressed in kg, A = area of test coupon in square meter, D = Density of the material in kg. m-1) at 283, 293, 303, 312 and 3230K temperatures and time mentioned 3, 5, 8, 11 and 14days. Their corrosion rate recorded in table1 and the results of table1 indicated that in marine water corrosion rate of polybutadiene-coated mild steel increased but its values decreased with the nanocoating of nanocoated and filler compounds. The plot between K(mmpy) versus t(days) in figure1 confirmed the above mentioned trends. The corrosion rate of poybutadiene increased with nanocoated octahydrobenzo[a,d][8]dihydrazone but this decreased with MgS filler, it observed different intervial of times. Polybutadiene-coated mild steel face sever crevice corrosion problem due to depletion of O2 inside and outside of polybutadiene. The use of nanocoating of octahydrobenzo[a,d][8]dihydrazone and MgS filler create composite thin film barrier which is more stable in marine water. This barrier is thermally stable and suppresses the attack of corrosive ions. MgS is active compounds which are entered into porosities of octahydrobenzo[a,d][8]annulene5,12-dihydrazone and forms complex this nitrogen containing compound. This surface film attaches with base materials by chemical bonding and forms a passive layer.
Table1. Corrosion rate of polybutadiene-coated mild steel nanocoated with octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone [NC(1)]and MgS filler in SO2 environment


There was Studied the effect of temperatures at 283, 293, 303, 313 and 3230K on polybutadiene-coated mild steel and after nanocoated with octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and MgS filler, it was observed that with the rise of temperatures corrosion enhanced but nanocoating and filler compounds reduced their values. This effect clearly depicted in table1 and figure2 which plotted between logK verse 1/T.

The values of log(ɵ/1-ɵ) for octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and MgS filler mentioned in table1 at different temperatures, it was found that log (ɵ/1-ɵ) decreased with nanocoating material as increasing temperatures but its values increased with filer as shown in table1 and figure3 which plotted between log(ɵ/1-ɵ) versus 1/T. The nanocoating and filler compounds developed a non osmosis barrier that neutralized the attack of Cl– ions and H2CO3.

The surface coverage areas were covered by octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and MgS filler at various temperatures and calculated by formula θ = (1- K / Ko) (where K is the corrosion rate before coating and Ko is the corrosion rate after coating) and their values were written in table1. It was observed that nanocoating compound increased surface coverage area but filler improved the mitigation character of surface coverage area. Such types of trends noticed in figure4 which plotted between ɵ(surface coverage area) versus T(temperature).

The percentage coating efficiency of octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and MgS filler were calculated by formula, %CE = (1 – K / Ko) X 100 (where CE = coating efficiency, K = Corrosion rate with coating, Ko = corrosion rate without coating) and its values were mentioned in table1. These results show that nanocoating and filler compounds increased coating efficiency but filler enhanced more efficiency. Figure5 plotted between %CE (percentage coating efficiency) versus T (temperature), it was observed that MgS filler increased coating efficiency of nanocoating compound in marine water.

Activation energy of polybutadiene-coated mild steel, octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and MgS filler were calculated by figure4.8.2 and Arrhenius equation, d /dt (logK) = Ea / R T2 (where T is temperature in Kelvin, R is universal gas constant and Ea is the activation energy of the reaction). The values of activation energies were expressed in table2 and plot between logK versus 1/T found to be straight lines in figure2. The positive values of activation energy indicated that nanocoated and filler compounds developed a thin film by chemical bonding. At higher temperature, activation energy reduced so both compounds formed stable barrier on the surface of base material.
Heat of adsorption of octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and MgS filler were obtained by Langmuir equation, log (θ/ 1-θ) = log (A .C) – (q/ 2.303R T)( where T is temperature in Kelvin and q heat of adsorption) and figure4.8.3 a linear graph between log (θ/ 1-θ) verse 1/T. Their values mentioned in table2 confirmed that nanocoating and filler compounds attached with base material by chemical bonding.
Free energy of octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and MgS filler were determined by formula, ΔG = -2.303RT log (33.3K) (where R is universal gas constant, T be temperature and K corrosion rate) and their recorded values in table2 gave information during coating exothermic reaction occurred. The results of table2 noticed that nanocoating and filler compounds were adhered with base material by chemical bonding.
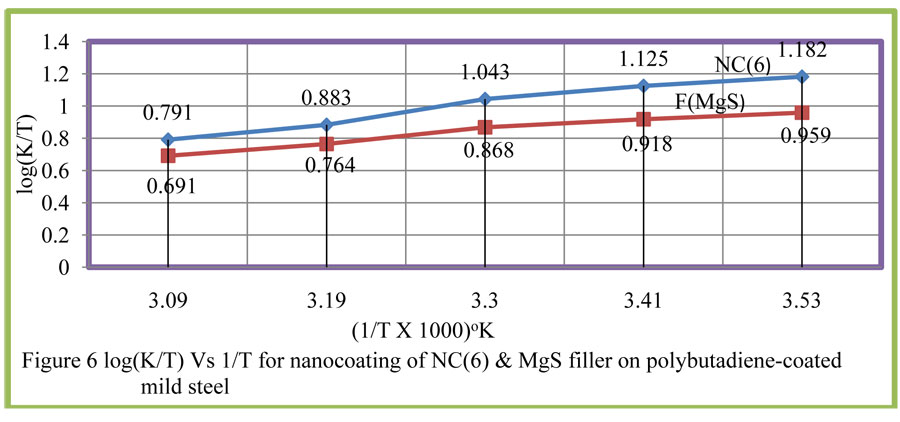
Enthalpy and entropy of octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and MgS filler were calculated by transition state equation, K = R T / N h log (ΔS# / R) X log (-ΔH #/ R T) (where N is Avogadro’s constant, h is Planck’s constant, ΔS# is the change of entropy activation and ΔH # is the change of enthalpy activation) and figure6 a linear graph between log(K/) versus 1/T and their values were written in table2. Nanocoating and filler compounds exhibited negative values of enthalpy and entropy. This sign indicated that coating is an exothermic process. Nanocoating and filler compounds were accommodated on the surface of base material by chemical bonding. Entropy values determined that filler compound arranged into matrix of nanocoating compound in ordered manner. Enthalpy and entropy of nanocoating of octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and ZnS filler indicate that the coating of both compounds is exothermic process.
Table2 Thermal parameter of octahydrodibenzo[a,d][8]annulene-5,12-dihyrazone and MgS filler nanocoating on polybutadiene-coated mild steel in marine water
| Thermal Parameters | 2830K | 2930K | 3030K | 3130K | 3230K |
| Ea(0) | 133.43 | 134.44 | 136.10 | 139.19 | 132.23 |
| Ea, NC(1) | 90.42 | 90.32 | 98.61 | 99.34 | 98.83 |
| q .,NC(1) | -35.25 | -34.76 | -29.23 | -26.85 | -25.01 |
| ∆G,NC(1) | -193.24 | -191.71 | -194.72 | -192.30 | -188.86 |
| ∆H, NC(1) | -53.45 | -57.64 | -65.88 | -68.65 | -69.90 |
| ∆S, NC(1) | -69.88 | -73.12 | -78.96 | -81.80 | -83.74 |
| θ, NC(1) | 0.7692 | 0.7735 | 0.7453 | 0.7338 | 0.7275 |
| Ea, F(MgS) | 84.21 | 84.61 | 87.55 | 86.77 | 85.65 |
| q .,F(MgS) | -43.08 | -44.42 | -43.31 | -42.90 | -42.02 |
| ∆G, F(MgS) | -187.03 | -183.96 | -183.67 | -179.73 | -175.65 |
| ∆H, F(MgS) | -47.23 | -49.89 | -54.83 | -56.08 | -56.69 |
| ∆S, F(MgS) | -66.36 | -68.57 | -72.27 | -73.92 | -75.19 |
| θ, F(MgS) | 0.8132 | 0.8276 | 0.8298 | 0.8349 | 0.8371 |
The results of all thermal parameters like activation energy, heat of adsorption, free energy, enthalpy and entropy at different temperatures were written in table 2 and their graph plotted in figure 7. After analysis of the results of all thermal parameters, it was found that surface coverage area increased as temperatures enhanced. The nanocoating and filler compounds formed thin surface film barrier on polybutadiene by chemical bonding that barrier stopped osmosis or diffusion process of marine water.

Potentiostat polarization results of polybutadiene-coated mild steel, octahydrodibenzo[a,d][8]annulene-5,12-dihydrazine and MgS filler were calculated by formula, ∆E/∆I = βa βc / 2.303 Icorr (βa + βc) (where ∆E/∆I is the slope which linear polarization resistance (Rp), βa and βc are anodic and cathodic Tafel slope respectively and I is thecorrosion current density in mA/cm2 ) and Tafel plot between ∆E(mV, electrode potential) versus I(mA/cm2, corrosion current density) in figure8 and their values were recorded in table3. It was observed that electrode potential and anodic current density was high with polybutadiene-coated mild steel but these values decreased with nanocoaing and filler compounds. Both compounds enhanced cathodic current density and reduced electrode potential. Corrosion current of polybutadiene-coated mild, octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and MgS filler were obtained by above equation and their values were put in equation, C. R (mmpy) = 0.1288 Icorr (mA /cm2) × Eq .Wt (g) / ρ (g/cm3) (where Icorr is the corrosion current density ρ is specimen density and Eq.Wt is specimen equivalent weight) produced corrosion rate.
The corrosion rate of all three materials were given in table3, it was observed that corrosion rate of polybutadiene-coated mild steel were high but these values were decreased with nanocoating and filler compounds. MgS filler enhanced cathodic current density and percentage coating efficiency with respect of octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone. Filler compound reduced more corrosion rate with octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone. Nanocoating and filler form a composite thin film on the surface of polybutadiene-coated mild steel during coating and its stability is good in hostile marine water. The results were obtained by weight loss experiment for polybutadiene-coated mild steel in marine water by nanocoating of octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and MgS were tally with the results of potentiostat.
Table3 Potentiostatic polarization of nanocoating octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and MgS filler on polybutadiene-coated mild steel.
| NC | ΔE (mV) | ΔI | βa | βc | Icorr (mA/cm2) | K (mmpy) | ᶿ | % CE | C (mM) |
| NC(0) | -451 | 112 | 172 | 151 | 8.69 | 264.69 | 0 | 0 | 0.0 |
| NC(1) | -311 | 65 | 75 | 18 | 4.81 | 146.51 | 0.71 | 71 | |
| F(MgS) | -251 | 55 | 65 | 195 | 4.65 | 141.63 | 0.88 | 88 |

Conclusion
Octahydrodibenzo[a,d][8]annulene-5,12-dihydrazone and MgS filler used for the corrosion protection of polybutadiene-coated mild steel. These materials formed coating composite coating barrier on the surface of base polybutadiene-coated mild steel. Nanocaoting compounds and filler compounds thermals parameters were indicated that composite surface barrier creation was exothermic process. The corrosive environment did not produce corrosion cell. The coating efficiencies and surface coverage area of nanocoating and filler compounds enhanced in different temperatures, atmosphere and weather changes.
Acknowledgement
Authors thankful for UGC-New Delhi provided financial support for this work. I give my thanks to laboratory staffs for their supports during experimental work.
References
- Bhadra S, Singh N K and Khastgir D (2011), Polyaniline based anticorrosive and anti-molding coating, Journal of Chemical Engineering and Materials Science Vol.2(1) 1-11.
- Szabo T, Molnar-Nagy L, and Telegdi J (2011), Self-healing microcapsules and slow release microspheres in paints, Progress in Organic Coatings, 72, 52-57.
- Wen N T, Lin C S, Bai C Y, and Ger M D (2008) Structures and characteristics of Cr (III) based conversion coatings on electrogalvanized steels, Surf. Coat. Technol, 203, 317.
- Boerio F J, Shah P (2005), Adhesion of injection molded PVC to steel substrates, J of Adhesion 81(6) 645-675.
- Deveci H, Ahmetti G and Ersoz M, (2012), Modified styrenes: Corrosion physico-mechanical and thermal properties evaluation, Prog. Org. Coat. 73 1-7.
- Genzer J (2005), Templating Surfaces with Gradient Assemblies, J of Adhesion 81 417-435.
- Leon-Silva U, Nicho M E (2010), Poly(3-octylthiophhene) and polystyrene blends thermally treated as coating for corrosion protection of stainless steel 304, J. Solid State Electrochem, 14 1487-1497.
- Baier R E (2006) Surface behaviour of biomaterials: Surface for biocompatibility, J. Mater. Sci. Mater. Med. 17 1057-1062.
- Rao BVA, Iqbal M Y and Sreehar B (2010), Electrochemical and surface analytical studies of the self assembled monolayer of 5-methoxy-2-(octadeclthiol) benzimidazole in corrosion protection of copper, Electrochim, Acta, 55 620-631.
- Liu X Y, Ma H Y and Hou M Z (2009), Self-assembled monolayers of stearic imidazoline on copper electrodes detected using electro chemical measurement, XPS, molecular simulation and FTIR, Chinese Sci. Bull. 54 374-381.
- Liao Q Q, Yue Z W and Zhou Q (2009), Corrosion inhibition effect of self-assembled monolayers of ammonium pyrrolidine dithiocarbamate on copper, Acta Phys. Chin. Sin. 25 1655-1661.
- Zhang D Q, He X M and Kim G S (2009), Arginine self-assembled monolayers against copper corrosion and synergetic effect of iodide ion, J. Appl. Electrochem 39 1193-1198.
- Sahoo R R and Biswas S K (2009), Frictional response of fatty acids on steel, J. Colloid Interf. Sci. 333 707-718.
- Raman R and Gawalt E S (2007), Selfassembled monolayers of alkanoic acid on the native oxide surface of SS316L by solution deposition, Langmuir, 23 2284-2288.
- Li D G, Chen S H and Zhao S Y (2006), The corrosion Inhibition of the self-assembled Au and Ag nanoparticles films on the surface of copper, Colloid. Surface. A 273 16-23.
- Cristiani P, Perboni G and Debenedetti A (2008), Effect of chlorination on the corrosion of Cu|Ni 70|30 condenser tubing, Electrochim. Acta 54 100-107.
- Cristiani P (2005), Solutions fouling in power station condensers, Appl. Therm. Eng. 25 2630-2640.
- Videla H and L K Herrera (2009), Understanding microbial inhibition of corrosion, Electrochem Acta, 39 229-234.
- Bibber J W (2009), Chromium free conversion coating for zinc and its alloys, Journal of Applied Surface Finishing, Vol. 2(4) 273-275.
- Ghareba G S and Omanovic S (2010), Interaction of 12-aminododecanoic acid with a carbon steel surface: Towards the development of ‘green’ corrosion inhibitors, Corrosion Sci. 52 2104-2113.
- Singh R K (2016), Corrosion protection of transport vehicles by nanocoating of decahydrobenzo[8]annulene-5,10-dihydrazone and SiC filler in H2O(moist), CO2, SO2 environments and weather change, Journal of Metallurgy and Materials Science, 58 167-179.
- Singh R K (2017), Corrosion protection of transport vehicles by nanocoating of decahydrobenzo[8]annulene-5,10-dihydrazone in corrosive environments and weather change, Journal of Powder Metallurgy & Mining, 1 2-8.
- Singh R K (2017), Atmospheric corrosion protection of epoxy-coated stainless steel by nanocoating of decahydrobenzo[8]annulene-5,10-disemecarbazone and TiN filler, International J of NME 2(4) 17-32.
Authors:
- Rajesh Kumar Singh*, Department of Chemistry, Jagdam College, J P University, Chpara-84130, India.
- Manjay Kumar Thakur, Research Scholar, Department of Chemistry, Jagdam College, J P University, Chapra-841301, India.
- Parshun Kumar, Research Scholar, Department of Chemistry, Jagdam College, J P University, Chapra-841301, India.





