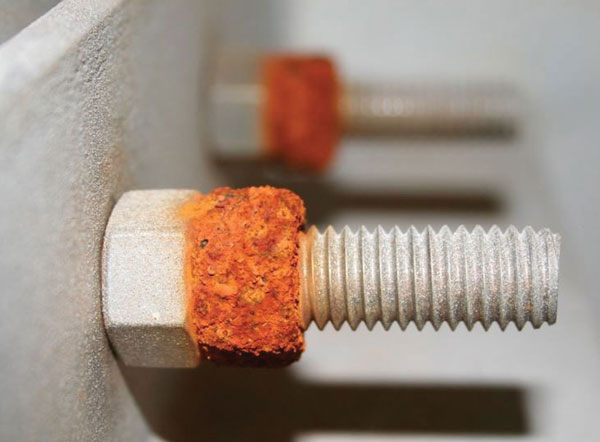
Corrosion, fire protection and fatigue failure of steel structures are some of the main concerns of an engineer involved in the design and construction of structural steelwork and these aspects do warrant extra attention. Corrosion is an electrochemical process involving an anode, a cathode, and an electrolyte. In the case of steel, when favourable conditions for corrosion occurs, the ferrous ions go into solution from anodic areas. Electrons are then released from the anode and move through the cathode where they combine with water and oxygen to form hydroxyl ions. These react with the ferrous ions from the anode to produce the hydrated ferrous oxide, which further gets oxidized into ferric oxide, which is known as the ‘red rust’.
Corrosion is both costly and dangerous. Billions of dollars are spent annually for the replacement of corroded structures, machinery, and components, including metal roofing, condenser tubes, pipelines, and many other items. In addition to replacement costs are those associated with maintenance to prevent corrosion, inspections, and the upkeep of cathodically protected structures.
Corrosion is of many types; however, we list relevant topics to Coated Carbon Steel Fasteners are as follows:
Rusting Corrosion
Most architectural fasteners are made from grades or alloys of steel that will react with oxygen to create ferrous oxide, commonly known as rust. Rust is a type of corrosion that weakens and deteriorates steel. Rusting is accelerated when steel is also exposed to moisture, especially if the moisture contains chlorides (salts), a condition that is common in marine, industrial, and urban atmospheres. When designing steel-to-steel connections, both the fasteners and the items being joined must be protected against rusting. Steel building panels, for example, can be protected by using zinc galvanizing and a high-performance coating.
Generalized corrosion (Oxygen type)
Uniform Surface Loss is the result of plain and low alloy steel containing lower than 13% of Chromium due to neutral water and in humid atmospheres. The water layer permits electrolytic reactions to develop on the steel surface, leading to progressive corrosion. The rate of corrosion rapidly increases in the presence of other pollutants and raised humidity levels.
Galvanic corrosion (hydrogen type)
Galvanic Corrosion occurs when dissimilar metals of equal surface area are in contact in the presence of an electrolyte (moisture), an electrical current is formed and the less noble metal migrates and dissolves into the solution. Galvanic corrosion is also used for corrosion prevention where a less noble metal (anode) i.e.zinc, is used to protect the cathode, carbon steel. However, where the coating is damaged its protective effect diminishes. Surface coatings on carbon steel fasteners are inevitably damaged during installation.
HASCC The Invisible Corrosion
A secondary effect of galvanic reaction can also lead to dangerous failure. Hydrogen, a by-product of galvanic corrosion, can weaken standard fasteners and cause failure. It produces a type of corrosion that is not readily apparent until it is too late. HASCC starts with hydrogen embrittlement.
Hydrogen embrittlement is associated with galvanic action. However, steel fasteners are not weakened by galvanic corrosion itself. Rather, hydrogen generated by galvanic action attacks the steel. Even if the steel is protected from galvanic corrosion, hydrogen can attack it rapidly. Specialized fasteners have been developed to avoid this risk.
Pitting corrosion
The anodic areas form a corrosion pit. This can occur with mild steel immersed in water or soil. This common type of corrosion is essentially due to the presence of moisture aided by improper detailing or constant exposure to alternate wetting and drying. This form of corrosion could easily be tackled by encouraging rapid drainage by proper detailing and allowing a free flow of air, which would dry out the surface.
Biochemical corrosion
Chemical corrosion is a process where Metals get dissolved in acids and caustic solutions of different strengths, which develops because metals tend to combine with oxygen to form oxides. This tendency is stronger the less noble the metal. The acids that attack fasteners often come from the atmosphere e.g. sulphuric acid, resulting from sulphur dioxide emissions from burning fossil fuels, is found in urban and industrial environments; nitric oxides, chlorine, hydrogen chloride, formic acid, acetic acid etc., are found in the vicinity of corresponding industrial plants; chloride and sodium chloride in particular, are common atmospheric pollutants in coastal regions.
Aeration cell corrosion
Thermal insulation leads to deficiency in Oxygen, Pollutants gather in laps of sheets where moisture is trapped. The area of restricted oxygen supply becomes the anode and corrosion will result, even in non-polluted areas of high pH value. A lower pH value i.e. where other pollutants are present, will increase the corrosion rate.
Crevice corrosion
The oxygen content of water trapped in a crevice is less than that of water which is exposed to air. Because of this, the crevice becomes anodic concerning surrounding metal and hence the corrosion starts inside the crevice.
Bimetallic corrosion
When two dissimilar metals (e.g. Iron and Aluminium) are joined together in an electrolyte, an electrical current passes between them and the corrosion occurs. This is because metals, in general, could be arranged, depending on their electric potential, into a table called the ‘galvanic series’. The farther the metals in the galvanic series, the greater the potential differences between them causing the anodic metal to corrode. A common example is the use of steel screws in stainless steel members and also using steel bolts in aluminium members.
Stress Corrosion
This occurs under the simultaneous influence of static tensile stress and a specific corrosive environment. Stress makes some spots in a body more anodic (especially the stress concentration zones) compared with the rest. This corrosion is not common with ferrous metals through some stainless steels are susceptible to this.
Fretting corrosion
If two oxide coated films or rusted surfaces are rubbed together, the oxide film can be mechanically removed from high spots between the contacting surfaces. These exposed points become active anodes compared with the rest of the surfaces and initiate corrosion. This type of corrosion is common in mechanical components.
Bacterial corrosion
This can occur in soils and water as a result of microbiological activity. Bacterial corrosion is most common in pipelines, buried structures and offshore structures
Hydrogen embrittlement
This occurs mostly in fasteners and bolts. The atomic hydrogen may get absorbed into the surface of the fasteners. When tension is applied to these fasteners, hydrogen will tend to migrate to points of stress concentration. The pressure created by the hydrogen creates and/or extends a crack. The crack grows in subsequent stress cycles. Although hydrogen embrittlement is usually included in the discussion about corrosion, actually it is not really a corrosion phenomenon.
Conclusion
In India there are no standards or Specifications of the Fasteners, hence we have to rely on foreign standards. The most detailed standards for fasteners are available with Australian Standards AS 3566 – 2002. This has last been updated in 2002. It is prescribed in two parts. Part 1 describes the Technical Specifications and Mechanical requirements of the fasteners, which defines how a fastener has to be made for Cold rolled steel and Hot Rolled Steel and Part 2 describes the Coating properties which a Fasteners must process hence that would avoid corrosion on fasteners when installed. The need to have part 2 for corrosion requirements arises when the same fasteners were used inside the building / in a moisture environment / in an exposed environment and near the sea. So, each manufacturer started giving different coatings and started their challenges. The pricing in the market was so difficult that the standards committee reached a point where they define.
EPDM – a synthetic polymer derived from oil – is the ideal material from which to manufacture fasteners. The number of ingredients in any given EPDM blend can range from 5 to 20 with hugely varying properties, such as UV stability, elasticity, compression resistance and resistance to heat aging. An important washer additive is carbon black. A small amount of carbon black is required in the EPDM blend as it offers important performance characteristics at a relatively low cost. The fundamental and generic considerations need to be recognized for any suitable rubber formulation. Formulation with a low level of carbon black is the way to go. Negligible electrical conductivity is also an important criterion. Fasteners of DEKS Industries meet all necessities required. DEKS EPDM Fasteners exhibit exceptional performance and provide compatibility with All metal Colour Coated ROOFING Sheets.