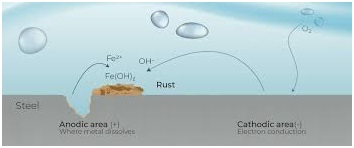
The corrosion process that takes place on a piece of uncoated steel is very complex. The corrosion of steel can be considered as an electrochemical process that occurs in stages. The initial attack occurs at anodic areas on the surface, where ferrous ions go into solution. Electrons are released from the anode and move through the metallic structure to the adjacent cathodic sites on the surface, where they combine with oxygen and water to form hydroxyl ions.. Factors such as variations in the composition/structure of the steel, presence of impurities due to the higher instance of recycled steel, uneven internal stress, and/or exposure to non-uniform environments all affect the corrosion process.
Corrosion is one of the highest concerns for industrial people. It affects steel as well as concrete surfaces under the abuse of acids, alkalies, chemicals, solvents, etc. Nearly US$ 5,000 is annual loss occurring globally due to loss of metal on account of corrosion. There are different types of steel corrosion and each of them has different structural effects, each of these is explained below.
TYPES OF STEEL CORROSION
Rusting Corrosion
Rust is a type of corrosion that weakens and deteriorates steel. Rusting is accelerated when steel is also exposed to moisture, especially if the moisture contains chlorides (salts), a condition that is common in marine, industrial, and urban atmospheres. When designing steel-to-steel connections, both the fasteners and the items being joined must be protected against rusting.
Generalized corrosion (Oxygen type)
Uniform Surface Loss is the result of plain and low alloy steel containing lower than 13% of Chromium due to neutral water and in humid atmospheres. The water layer permits electrolytic reactions to develop on the steel surface, leading to progressive corrosion. The rate of corrosion rapidly increases in the presence of other pollutants and raised humidity levels.

Galvanic corrosion (hydrogen type)
Galvanic Corrosion occurs when dissimilar metals of equal surface area are in contact in the presence of an electrolyte (moisture), an electrical current is formed and the less noble metal migrates and dissolves into the solution.

HASCC- The Invisible Corrosion
A secondary effect of galvanic reaction can also lead to dangerous failure. Hydrogen, a by-product of galvanic corrosion, can weaken standard fasteners and cause failure. It produces a type of corrosion that is not readily apparent until it is too late. HASCC starts with hydrogen embrittlement. Hydrogen embrittlement is associated with galvanic action. However, steel fasteners are not weakened by galvanic corrosion itself.

Pitting corrosion
The anodic areas form a corrosion pit. This can occur with mild steel immersed in water or soil. This common type of corrosion is essentially due to the presence of moisture aided by improper detailing or constant exposure to alternate wetting and drying. This form of corrosion could easily be tackled by encouraging rapid drainage by proper detailing and allowing a free flow of air, which would dry out the surface.

Biochemical corrosion
Chemical corrosion is a process where metals get dissolved in acids and caustic solutions of different strengths, which develops because metals tend to combine with oxygen to form oxides. This tendency is stronger the less noble the metal. The acids that attack fasteners often come from the atmosphere e.g. sulphuric acid, resulting from sulphur dioxide emissions from burning fossil fuels, is found in urban and industrial environments; nitric oxides, chlorine, hydrogen chloride, formic acid, acetic acid etc., are found in the vicinity of corresponding industrial plants; chloride and sodium chloride in particular, are common atmospheric pollutants in coastal regions.

Aeration cell corrosion
Thermal insulation leads to deficiency in Oxygen, Pollutants gather in laps of sheets where moisture is trapped. The area of restricted oxygen supply becomes the anode and corrosion will result, even in non-polluted areas of high pH value. A lower pH value i.e. where other pollutants are present, will increase the corrosion rate.

Crevice corrosion
The oxygen content of water trapped in a crevice is less than that of water which is exposed to air. Because of this, the crevice becomes anodic concerning surrounding metal and hence the corrosion starts inside the crevice.

Bimetallic corrosion
When two dissimilar metals (e.g. Iron and Aluminium) are joined together in an electrolyte, an electrical current passes between them and the corrosion occurs. This is because metals, in general, could be arranged, depending on their electric potential, into a table called the ‘galvanic series’.

Stress Corrosion
This occurs under the simultaneous influence of static tensile stress and a specific corrosive environment. Stress makes some spots in a body more anodic (especially the stress concentration zones) compared with the rest. This corrosion is not common with ferrous metals though some stainless steels are susceptible to this.

Fretting corrosion
If two oxide coated films or rusted surfaces are rubbed together, the oxide film can be mechanically removed from high spots between the contacting surfaces. These exposed points become active anodes compared with the rest of the surfaces and initiate corrosion. This type of corrosion is common in mechanical components.

Bacterial corrosion
This can occur in soils and water as a result of microbiological activity. Bacterial corrosion is most common in pipelines, buried structures and offshore structures

STRUCTURAL EFFECTS OF CORROSION
As the corrosion process progresses, corrosion products tend to build up in certain areas of the metal. These corrosion products have different elemental compositions than the original state. The new compositions exposed on the surface lead to changes in the anodic and cathodic areas. As the change in anodic and cathodic areas occur, previously uncorroded areas of the metal can be attacked and corrode. This will accelerate the overall corrosion of the steel surface. Discussed below are the structural effects of corrosion.
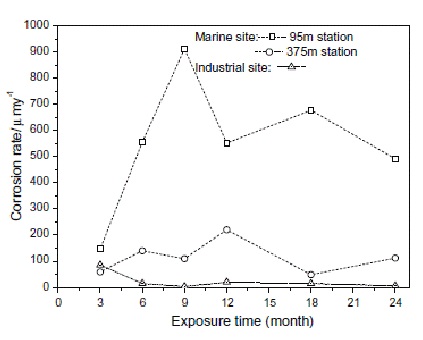
Image caption- Corrosion Rate of Carbon Steel in Different Atmosphere Conditions
- LOSS OF STRENGTH
Corrosion reduces the effective cross section of structural components. This will reduce the axial, and flexural strength of elements, and makes them structurally weak. Even if corroded elements look stable, it does not mean they are safe; in fact, the corroded structures become vulnerable for design loads (ultimate loads), i.e. a strong ground motion can increase the stress actions beyond the capacity of the sections. Loss of strength can happen in steel and reinforced concrete structures. Corrosion under insulation -CUI- is a frequent observation in refineries, and the oil and gas industry. Steel sections covered under fireproofing insulation experience corrosion over their service life. Another famous example is the reduced flexural, and shear capacity of the RC element.
- FATIGUE
Another structural effect of corrosion is on the fatigue strength of steel elements, connections, and RC elements. Corrosion may accelerate fatigue crack propagation in structural steels. Development of pitting corrosion introduces additional points of stress concentration at which cracking may develop, which will reduce the fatigue strength.
- REDUCED BOND STRENGTH
The capacity of composite elements such as RC elements depends on the characteristics of concrete-rebar interface. When steel corrodes, the products of corrosion expand. This will leave a poor quality steel layer over the surface of the reinforcement. This layer has a poor bond with surrounding concrete; therefore, it will reduce the capacity of the section. In case of lap splices or anchorage, this may reduce the effective length of anchorage, and resulting in premature failure of sections.
- LIMITED DUCTILITY
Corrosion can significantly reduce the ductility of RC sections. This is critical in seismic design and evaluation. Corroded sections have lower ductility, which means their plastic deformation is limited. This will affect the seismic response of the elements. Corrosion of reinforcement in the lap splices will affect the load transfer in the laps, preventing the to develop yield stress.
- REDUCED SHEAR CAPACITY
Corrosion can reduce the effective cross sectional area of transverse reinforcement in beams and columns, and reduce the shear capacity of the section. In concrete slabs, this can reduce the shear strength of the slab close to the columns, and increase the chance of punching shear failure. In footings, the corrosion can result in shear failure of the footing, anchorage failure, or flexural yielding of steel reinforcement.
CONCLUSION
Steel is a proven durable and efficient building material that has been used since the Industrial Revolution. It is cost effective, aesthetically pleasing, sustainable, and strong. However, like all metals, steel corrodes when exposed to the atmosphere. Therefore, it is important to consider corrosion protection methods when constructing projects with exposed steel. There are a number of different corrosion protection systems to consider including:
- Hot-Dip Galvanizing
- Duplex Systems
- Zinc Coatings
- Protective Coatings
- Special Steels
- Sacrificial Anodes
- EPDM – a synthetic polymer derived from oil
Image Source-steel fabrication seamless, researchgate,cathwelll, nity-grity, metallurgy for device, electrochemical society, science direct, bearing centre, azom.com, colorcorroison.com